UV-curable silicone-modified polyurethane combines the mechanical strength of polyurethane, the weather resistance of silicone and the high efficiency and environmental protection characteristics of UV curing, and has become a research hotspot for high-performance coatings, adhesives and electronic packaging materials .

uneven cross-linking caused by the easy hydrolysis of the silicon-oxygen chain segment:
1. Terminal alkylation protection technology
Introducing alkyl groups (such as methyl and butyl) at the ends of polydimethylsiloxane (PDMS) segments increases the molecular distance, reduces the contact between the silicon-oxygen segments and water, and significantly reduces the risk of hydrolysis. Alkylation also enhances hydrophobicity, making the coating water contact angle reach 128°–165°, close to a superhydrophobic state.

2. Synergistic effect of cyclic ether dilution monomer
Experiments show that this design allows the cured coating to maintain its intact structure after 500 hours of testing in an 85°C/85%RH environment. Cyclic ether acrylates (such as tetrahydrofuran acrylate) are added as active diluents, and their strongly electronegative oxygen atoms preferentially absorb environmental moisture to form a "moisture barrier" that protects the silicon-oxygen chain segments from hydrolysis.
3. Rigid-flexible segment composite
The introduction of polyaryletherketones (such as terminal olefin-based silicone polyaryletherketones) improves heat resistance through the rigid structure of the aromatic ring (the thermal decomposition temperature is increased by 30–50°C), while the polysiloxane chain segments provide flexibility (the elongation at break is increased by 40%).
2. Innovation of Photoinitiators: From Efficiency to Environmental Protection
Traditional photoinitiators have problems such as residual toxicity, oxygen inhibition and insufficient deep curing. The new initiator system has made breakthroughs in the following directions:
1. Development of special initiators for UV-LED
Macromolecular design (such as lignin-based photoinitiators) reduces small molecule migration and reduces toxicity to Draize 1 (skin irritation). Thioxanthone derivatives (such as S-2) increase absorption efficiency by 50% in the 365–405 nm wavelength range, are compatible with LED light sources, and reduce energy consumption by 60%.
2. Synergistic effect of composite initiation system
H⁺ after photolysis , catalyzing siloxane condensation to achieve deep curing in shadow areas. Free radical/cationic hybrid systems (such as Irgacure 819 and sulfonium salt UVI6992) simultaneously initiate acrylate polymerization and cationic ring-opening reactions, increasing the curing speed by 3 times and eliminating oxygen inhibition.
3. Application of low migration initiators
Hyperbranched macromolecular photoinitiators (such as acrylated nanocellulose) form covalent bonds with the polyurethane matrix, and the residual amount is <0.1%, meeting the standards for food packaging and medical devices.
3. Dual curing: Overcoming the bottleneck of curing complex structures
For shadow areas of three-dimensional structural parts (such as automotive PCB boards), light/dark dual curing becomes a key technology:
UV/Moisture Dual Cure
The modified polyurethane resin contains acrylic double bonds (UV curing) and isocyanate groups (–NCO), which form polyurea in contact with moisture, allowing the non-light-exposed areas to cure. The peel strength reaches 8.5 N/mm (120% higher than traditional systems).
Light/heat dual curing
Thermosensitive photoinitiators (such as acylphosphine oxide TPO) generate free radicals after UV irradiation, and the unreacted groups are further cross-linked at 80°C, with a curing degree of more than 95%.
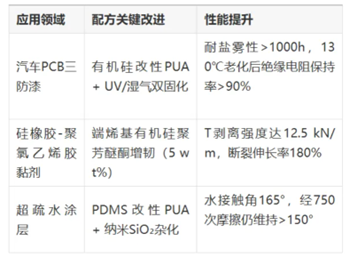
IV. Application Scenarios and Performance Verification
V. Challenges and Future Directions
Although fast-curing UV silicone-modified polyurethane photoinitiators show broad application prospects, they still face some technical difficulties and challenges in actual development and application :
1. Balance between photoinitiator efficiency and mobility : Although macromolecularization and silicone modification of photoinitiators reduce migration and toxicity, they may affect their light absorption and free radical generation efficiency.
As the molecular weight of the macromolecular initiator increases, the diffusion rate in the system decreases, which may lead to a decrease in initiation efficiency, requiring higher light intensity or longer time for complete curing.
In addition, the introduction of silicone segments may change the refractive index and light absorption characteristics of the system, and the structure and concentration of the photoinitiator need to be optimized to ensure sufficient light absorption and free radical yield. How to maintain high initiation efficiency while reducing mobility is one of the key challenges in this field.
2. Compatibility of silicone and polyurethane : The polarity of silicone (such as PDMS) and polyurethane is quite different, and direct blending is prone to phase separation, affecting the stability of the emulsion and coating performance. Early studies have found that simple physical blending of silicone will lead to emulsion instability, limiting the increase of silicone content.
Therefore, it is usually necessary to introduce silicone segments into the polyurethane main chain through chemical grafting or copolymerization to improve compatibility. However, the chemical modification process is complicated and the reaction conditions are harsh (such as the need to strictly control the ratio of isocyanate to hydroxyl group, reaction temperature, etc.), otherwise gelation or phase separation may occur.
How to improve the dispersion and stability of silicone in polyurethane to obtain a system with high silicone content and uniform performance is a major difficulty in the preparation process.
3. Control of cured film properties : While silicone modification brings low surface energy and flexibility, it may also have an adverse effect on certain properties of the coating. Too many silicone segments may reduce the hardness and adhesion of the coating.
Studies have shown that as the silicone content increases, the tensile strength of the coating decreases. Therefore, the silicone content needs to be weighed in the formulation design to achieve a balance of the required properties.
the cross-linking density and network structure of the silicone-modified polyurethane photoinitiator system are also relatively complex. How to obtain ideal hardness, flexibility, scratch resistance, etc. through formulation and process control is a problem that needs to be solved in practical applications.
4. Cost and large-scale production : The synthesis of organosilicon-modified polyurethane photoinitiators involves multiple chemical reactions, the cost of raw materials (such as terminal hydroxyl silicone oil, special diisocyanate, photoinitiator monomer, etc.) is relatively high, and the synthesis process is relatively complex. This makes the production cost of this type of photoinitiator higher than that of traditional small molecule initiators.
In addition, large-scale production requires consideration of the reproducibility and stability of the reaction , control of temperature uniformity in large-volume reactors, and avoidance of local gelation.
At present, most of the related research remains in the laboratory stage, and there are still many engineering problems to be solved to achieve industrial mass production. How to reduce costs and simplify processes is an important challenge to promote the industrialization of this technology.
5. In practical applications, the compatibility of the photoinitiator with other components must also be considered , such as compatibility with active diluents and additives, and the adaptability of the curing equipment .
Some silicone-modified systems may have a higher viscosity and require adjustment of the formula to meet construction requirements. For example, different light sources (mercury lamps, LEDs, etc.) have different emission spectra and require the photoinitiator to have good absorption in the corresponding band, which may require adjustment of the photoinitiator structure or the use of a composite initiator system.
Regulations and safety are also factors that need to be considered. New photoinitiators must pass toxicology and migration tests and comply with relevant food safety and environmental regulations before they can be used in food packaging, medical devices and other fields.
With the evolution of new photoinitiators towards high efficiency, low toxicity and broad spectrum, and the breakthrough of dual curing technology in complex working conditions, this material will be widely used in new energy battery packaging, flexible electronics and marine corrosion protection , and will promote UV curing technology to develop in the direction of higher performance and more environmentally friendly.